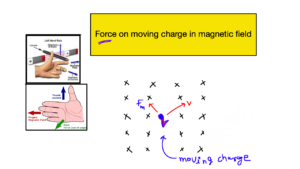
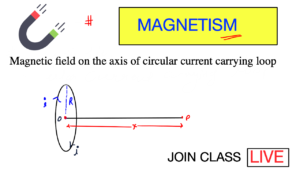
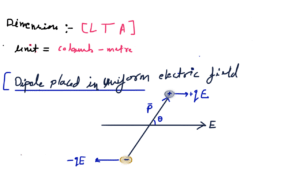
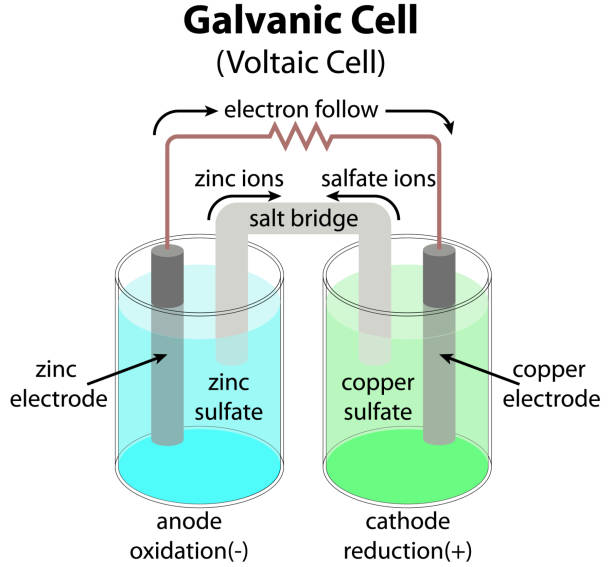
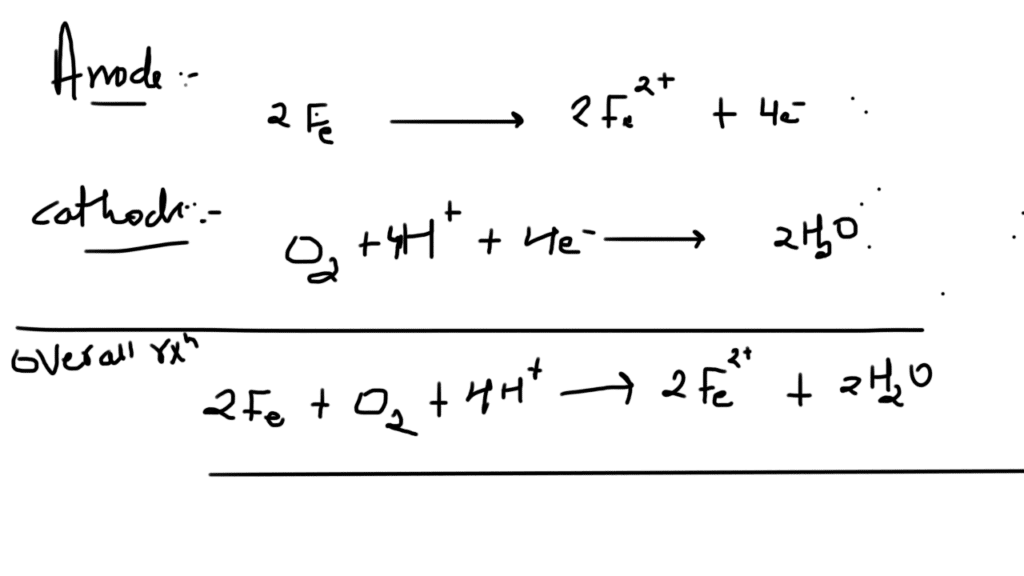Electrochemistry is a branch of chemistry that deals with the study between electricity and chemical reactions. It explores the transformation of chemical energy into electrical energy, and vice versa.
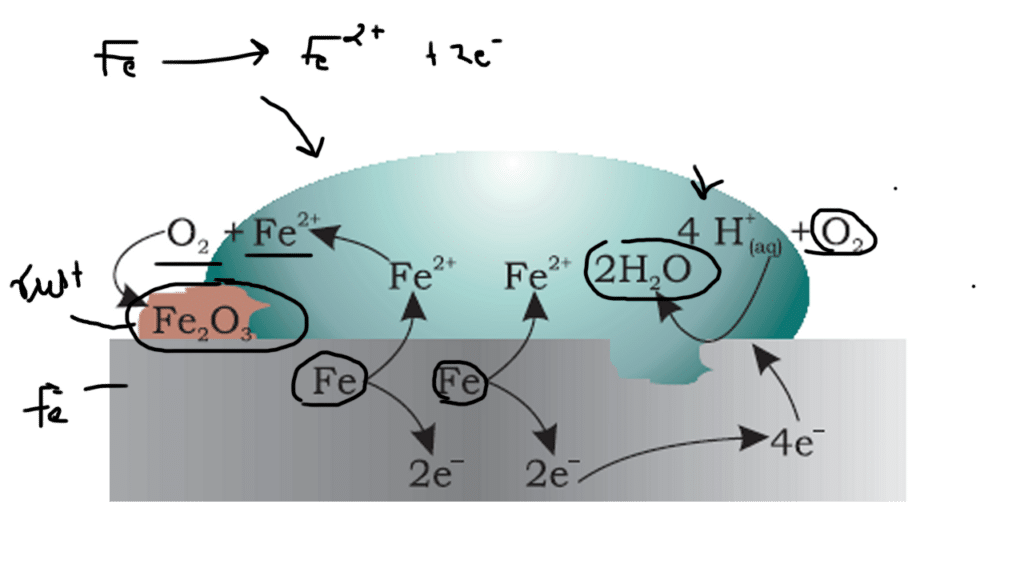
From the rusting of iron , batteries and fuel cells these all are the example of redox reactions , electrochemistry plays a crucial role in many aspects of our daily lives.

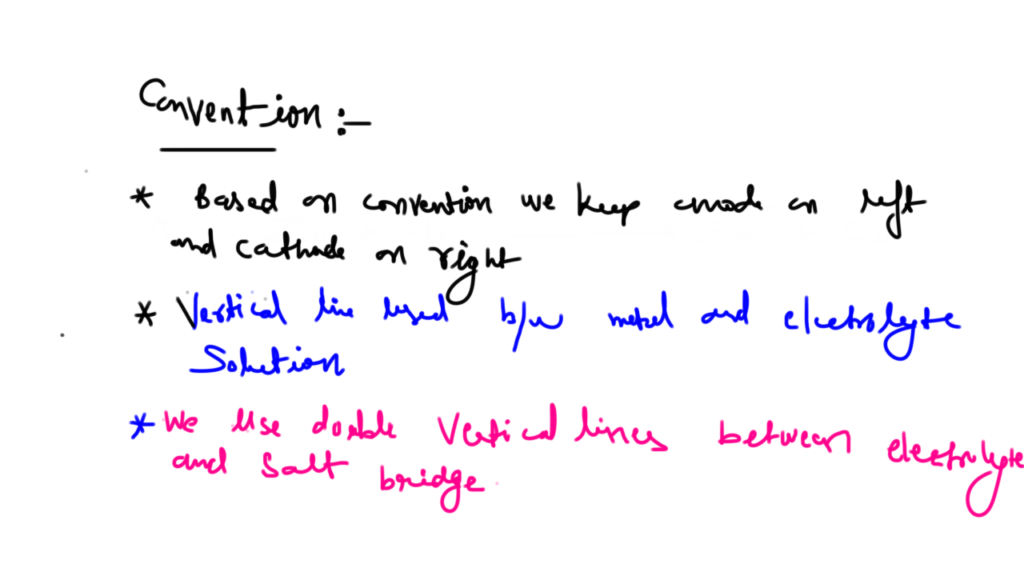
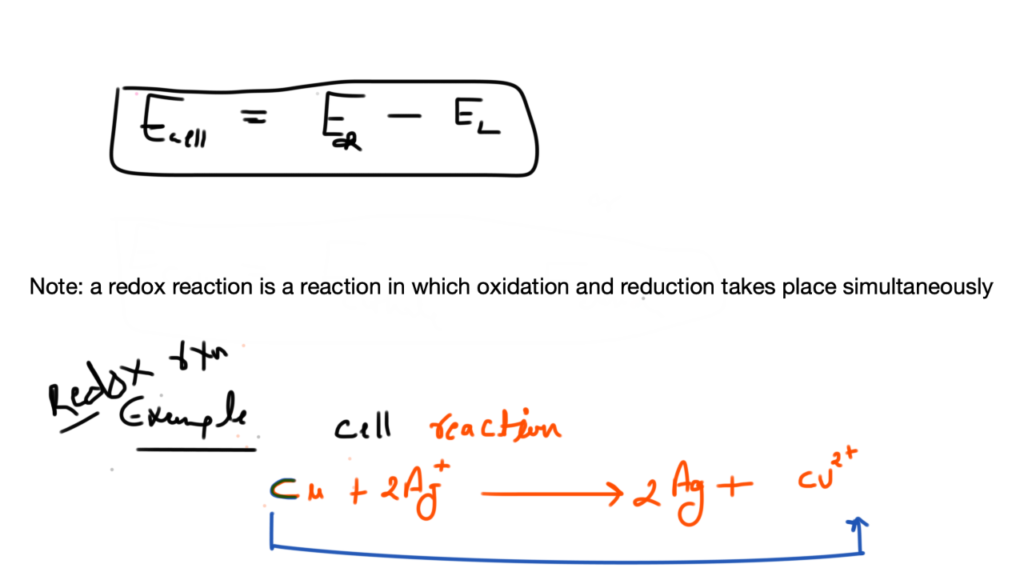
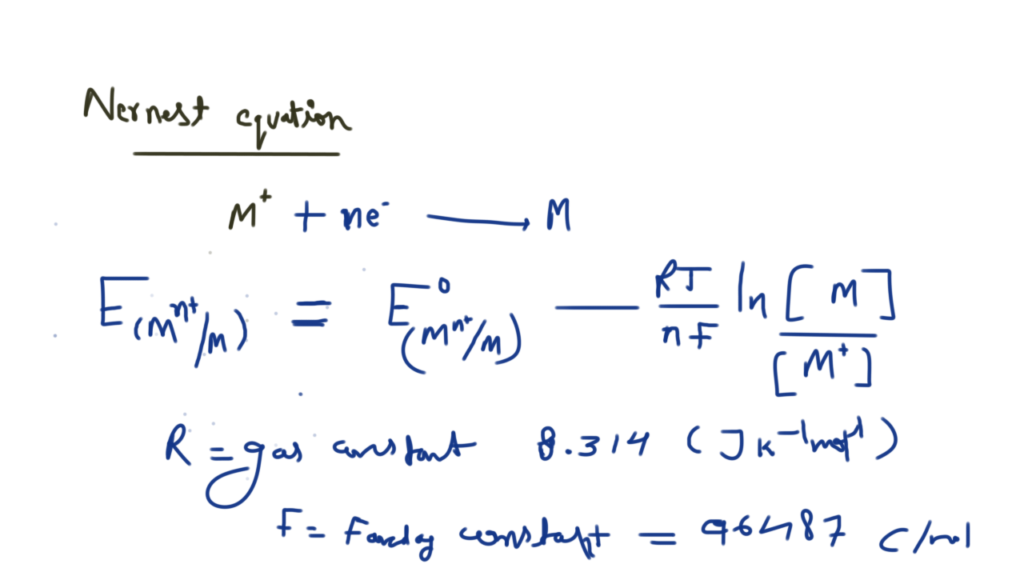
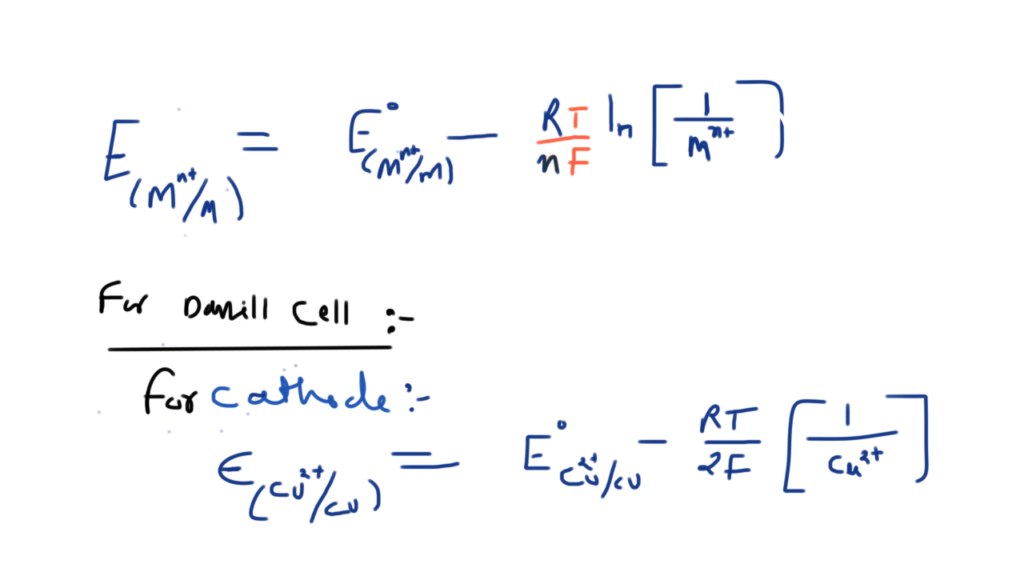
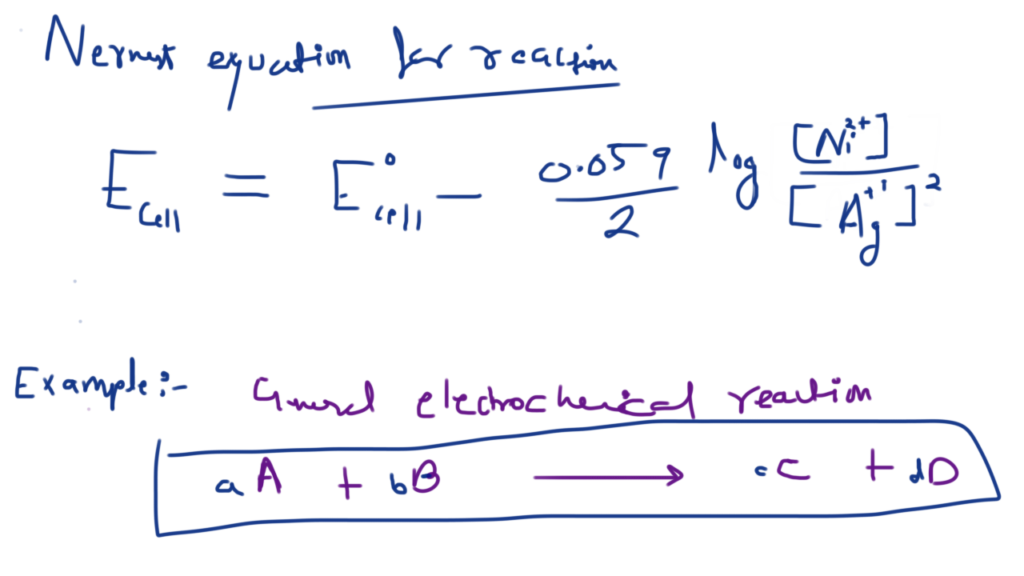
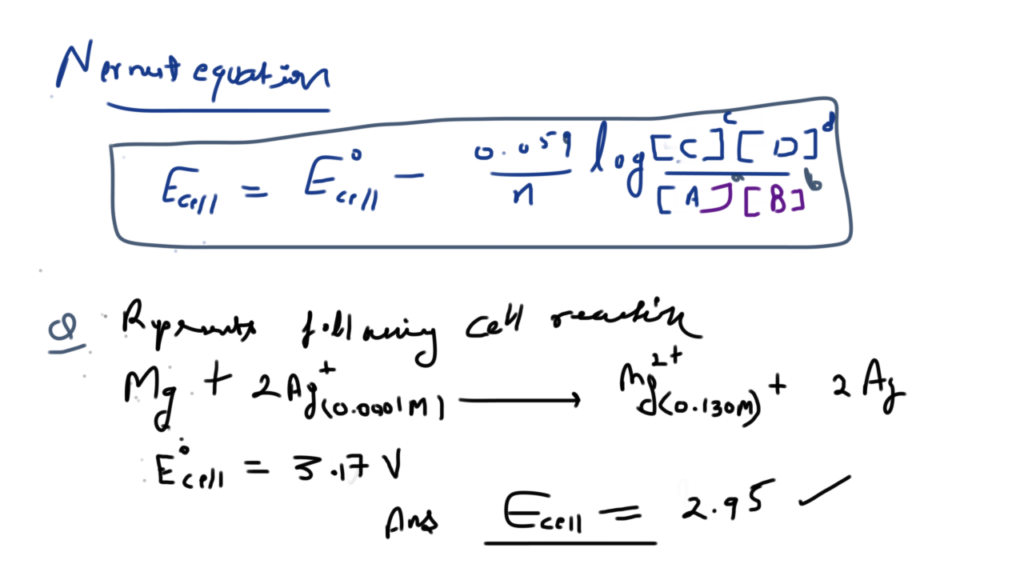


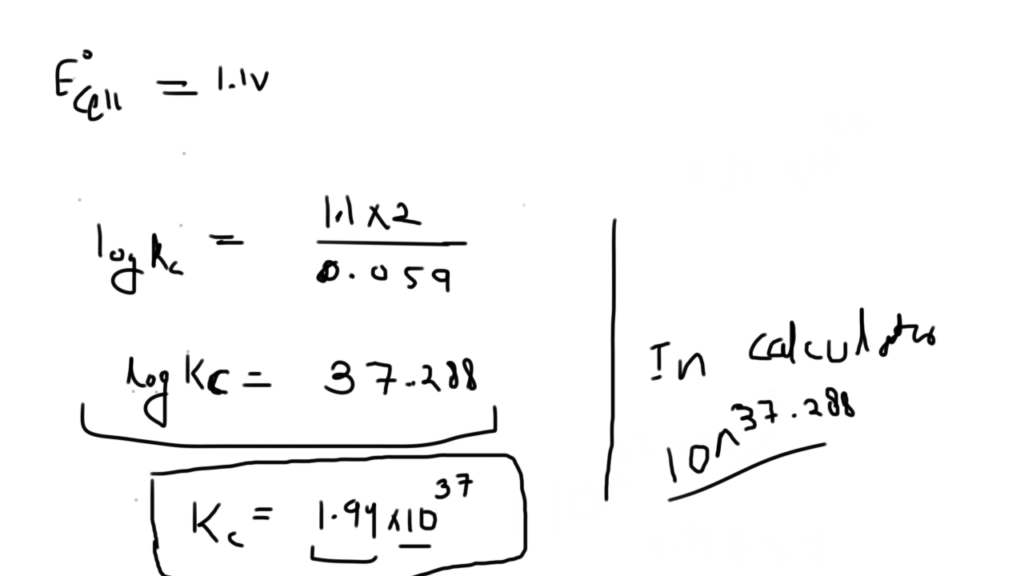
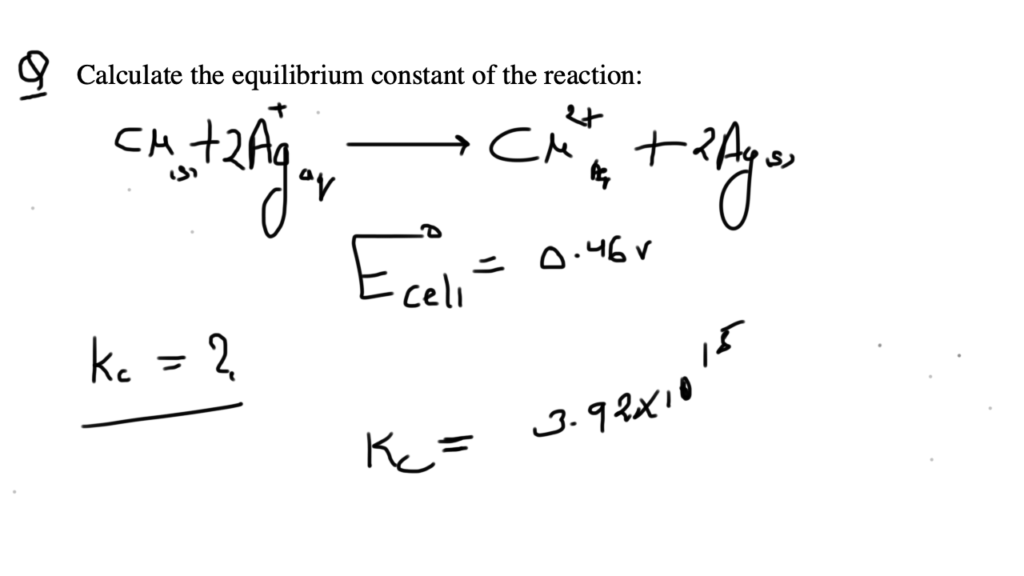
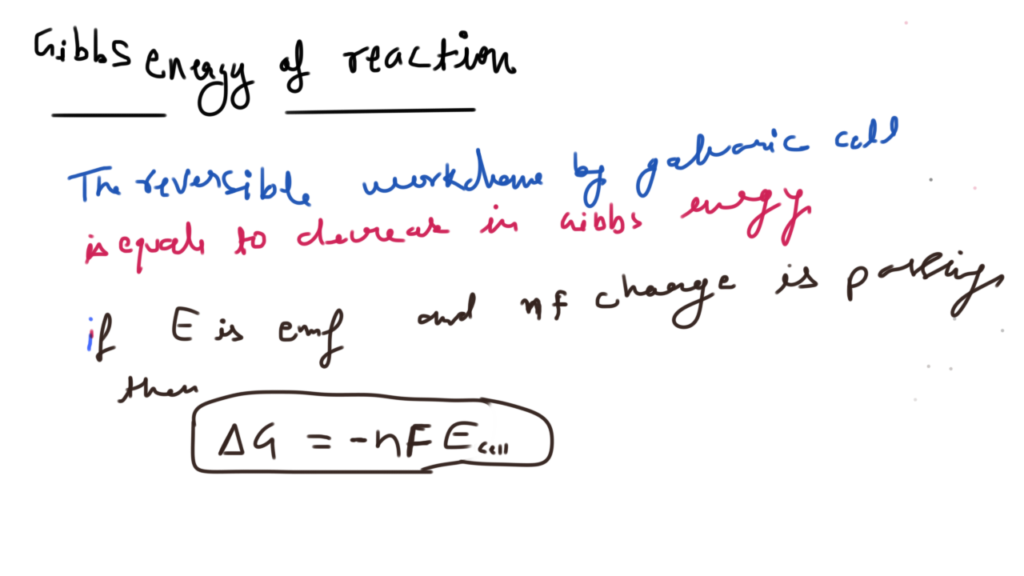
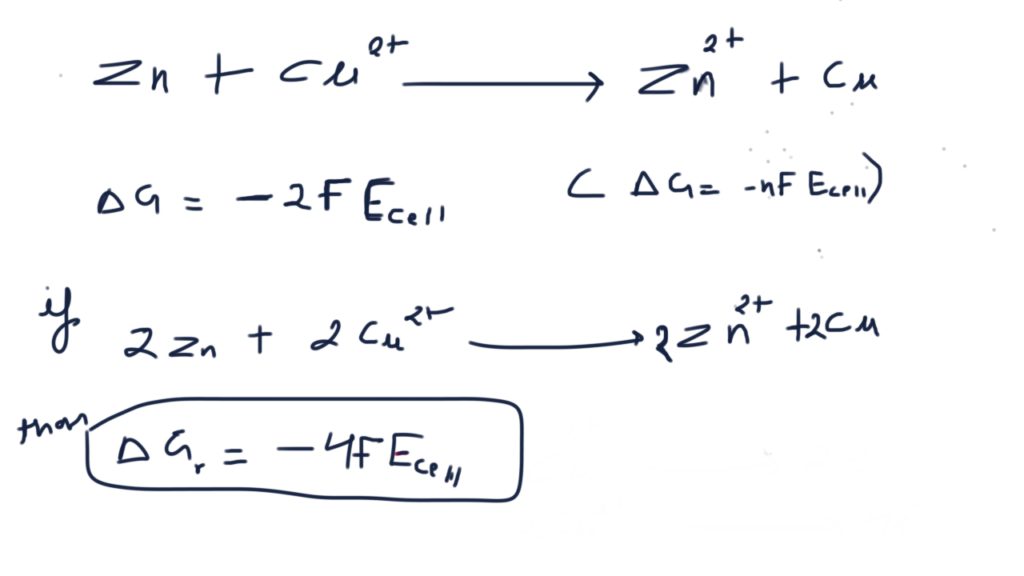
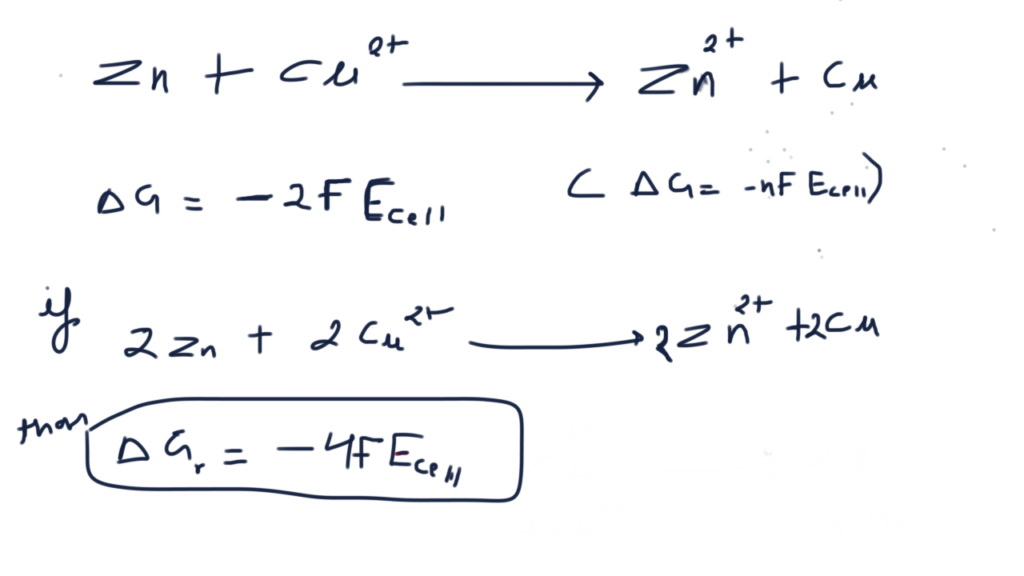
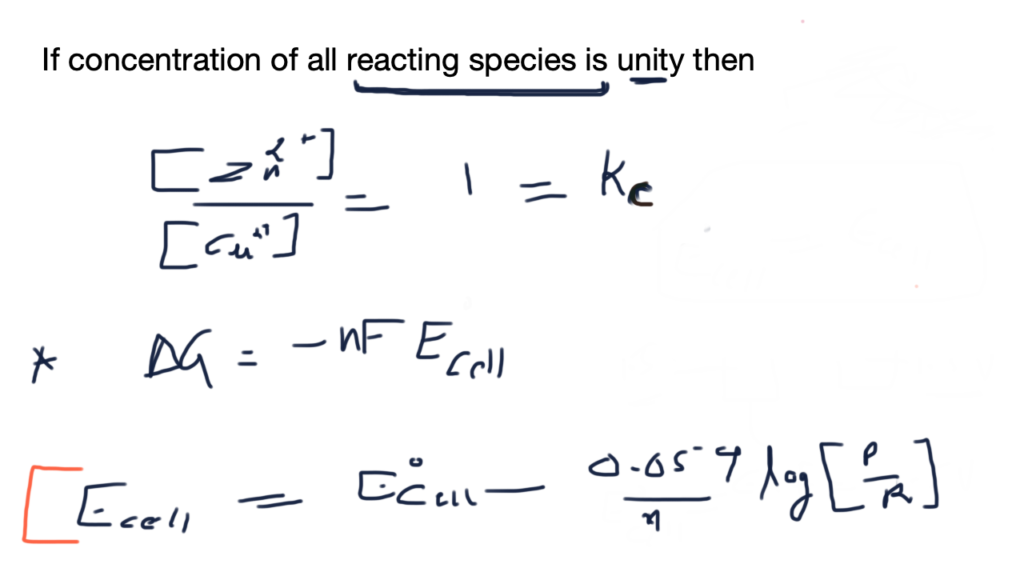






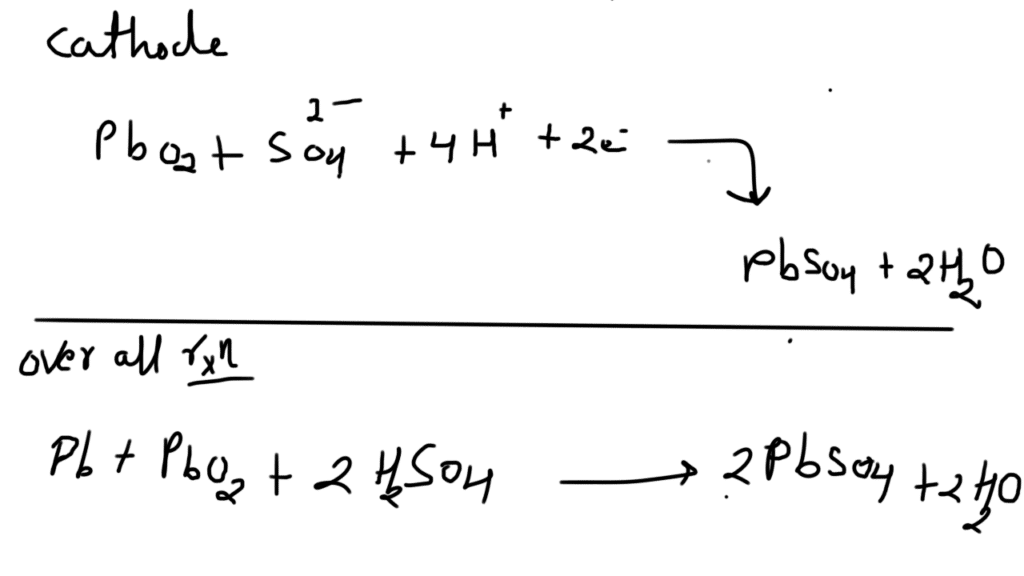
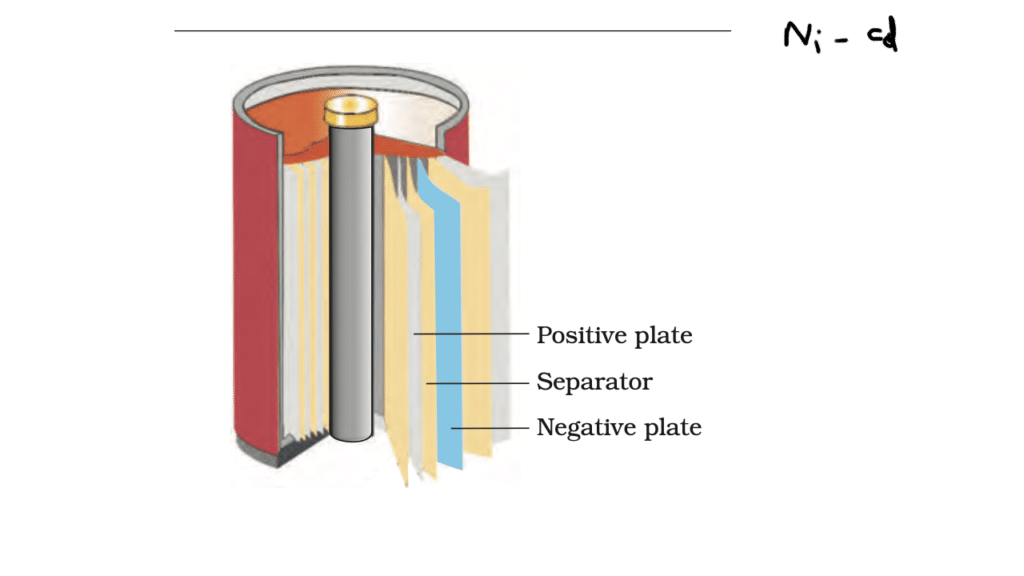
Nickel cadmium cell
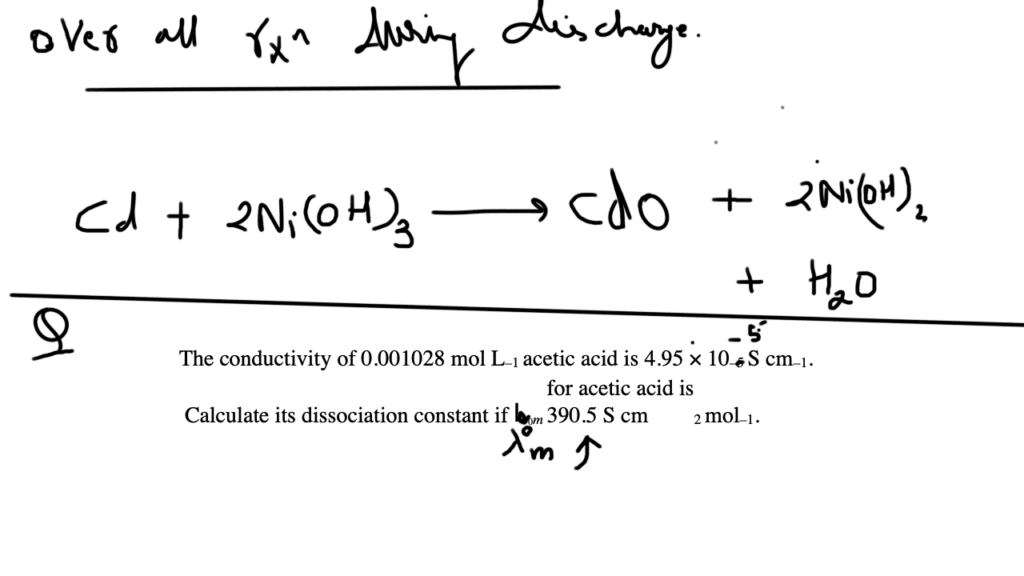
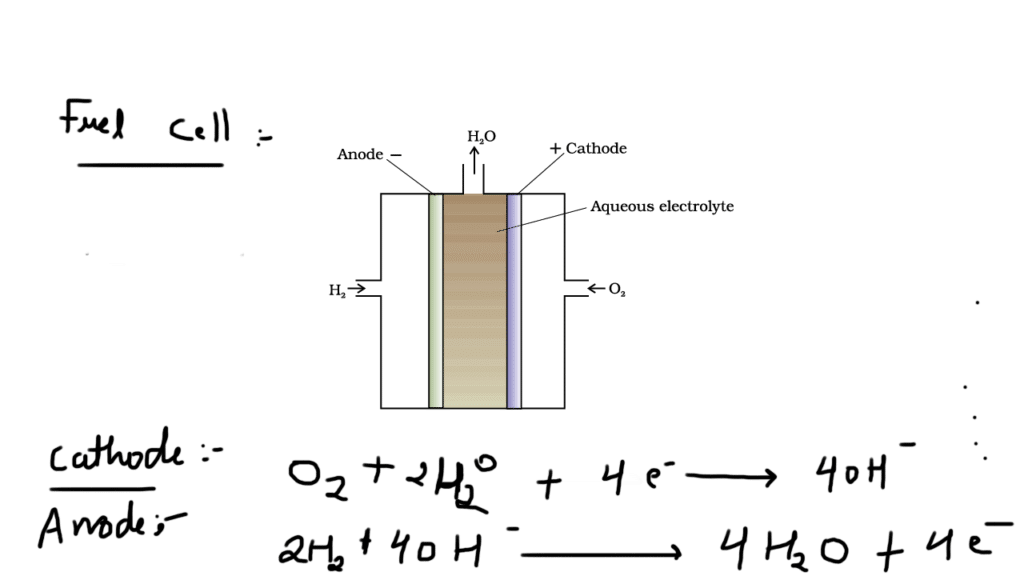
Fuel cell
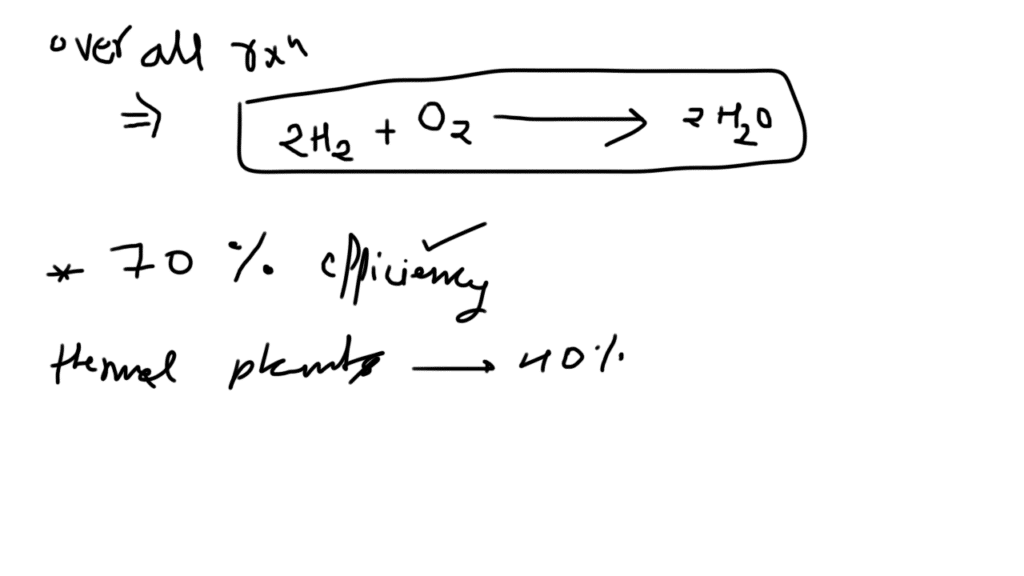
Rusting