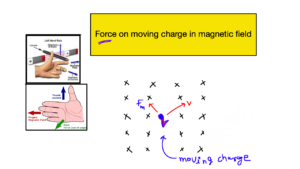
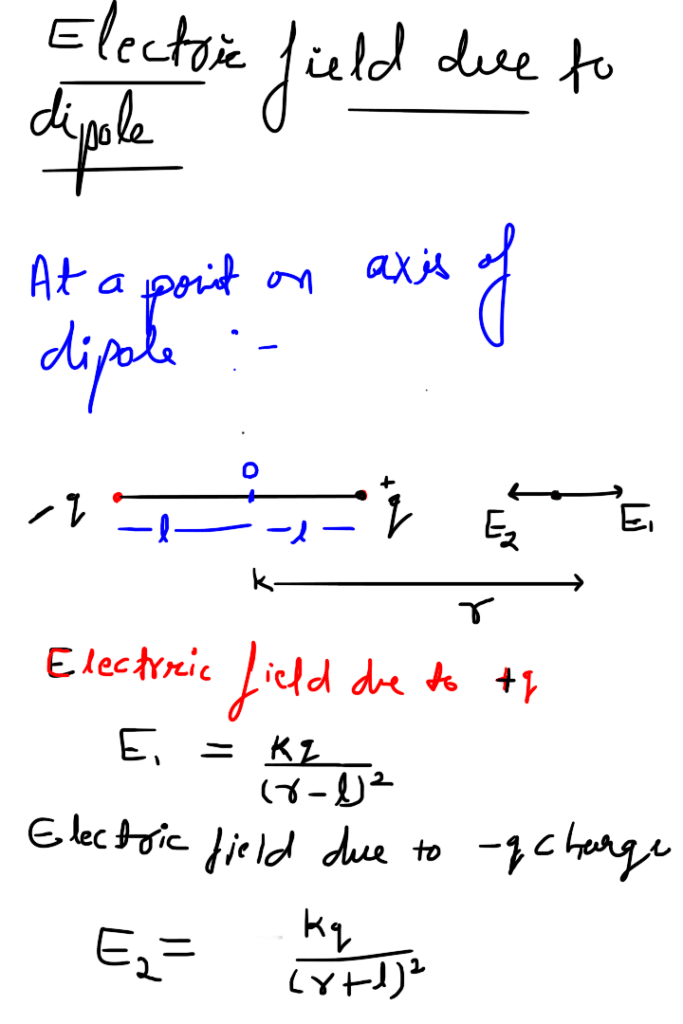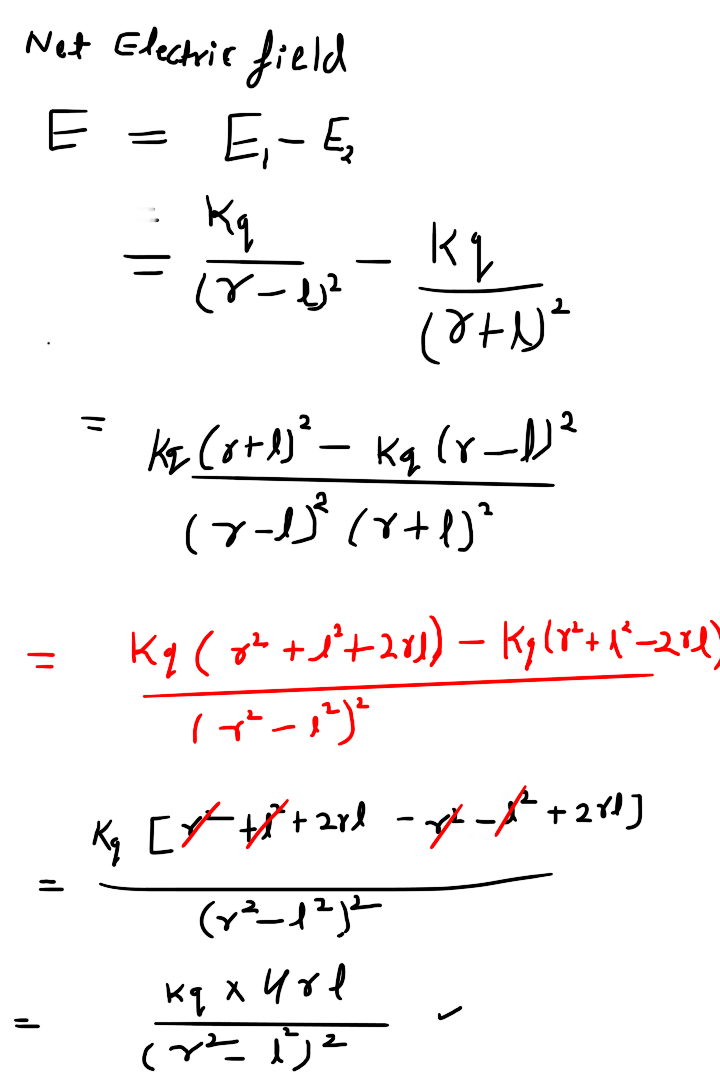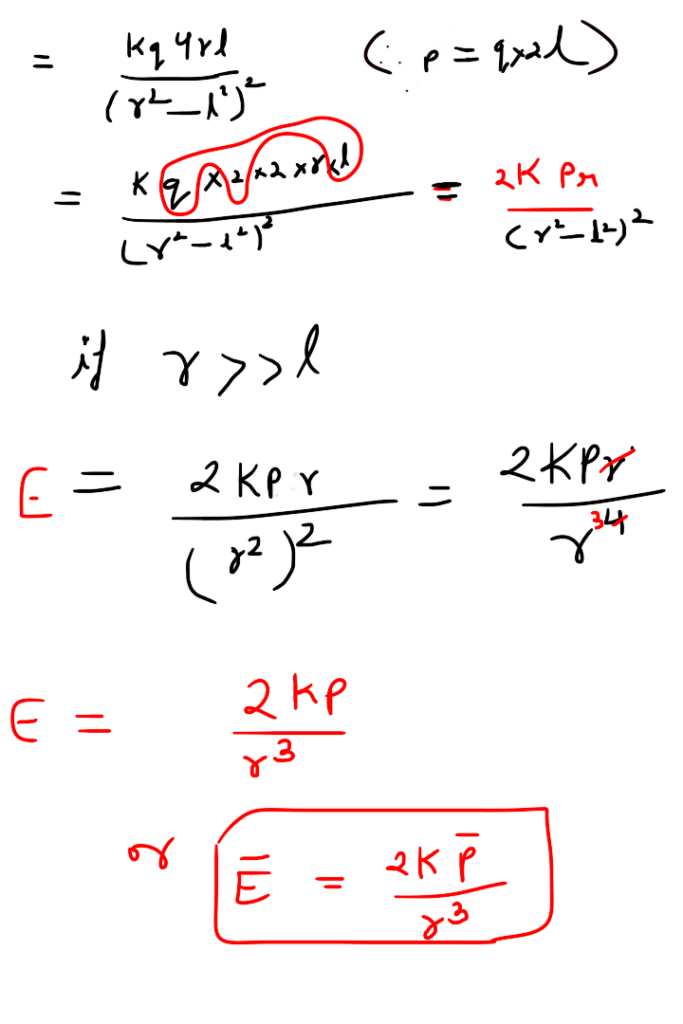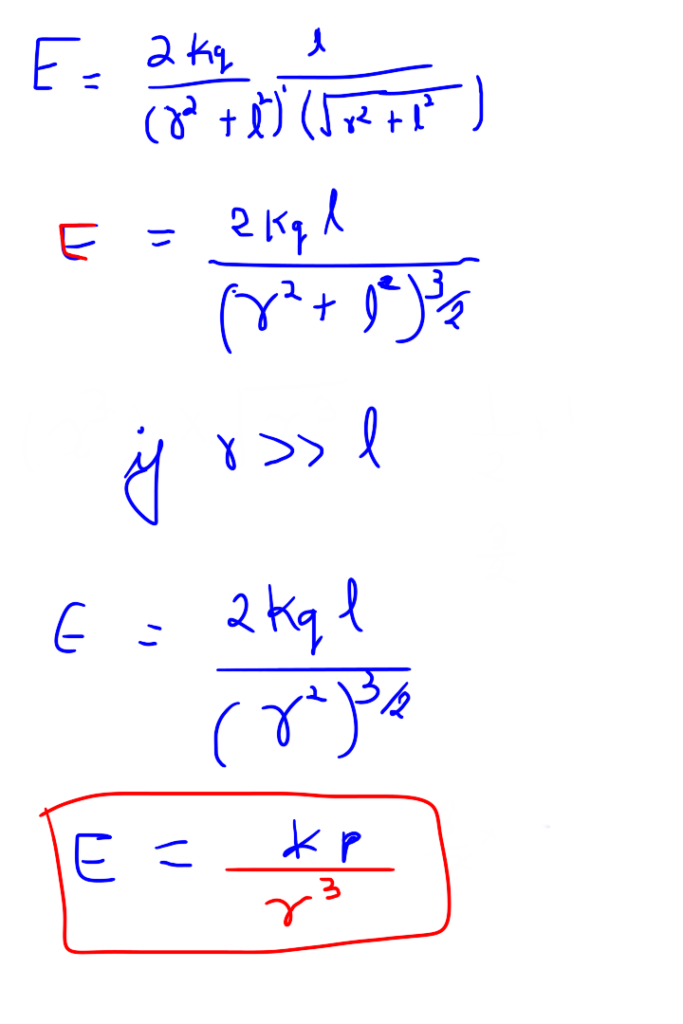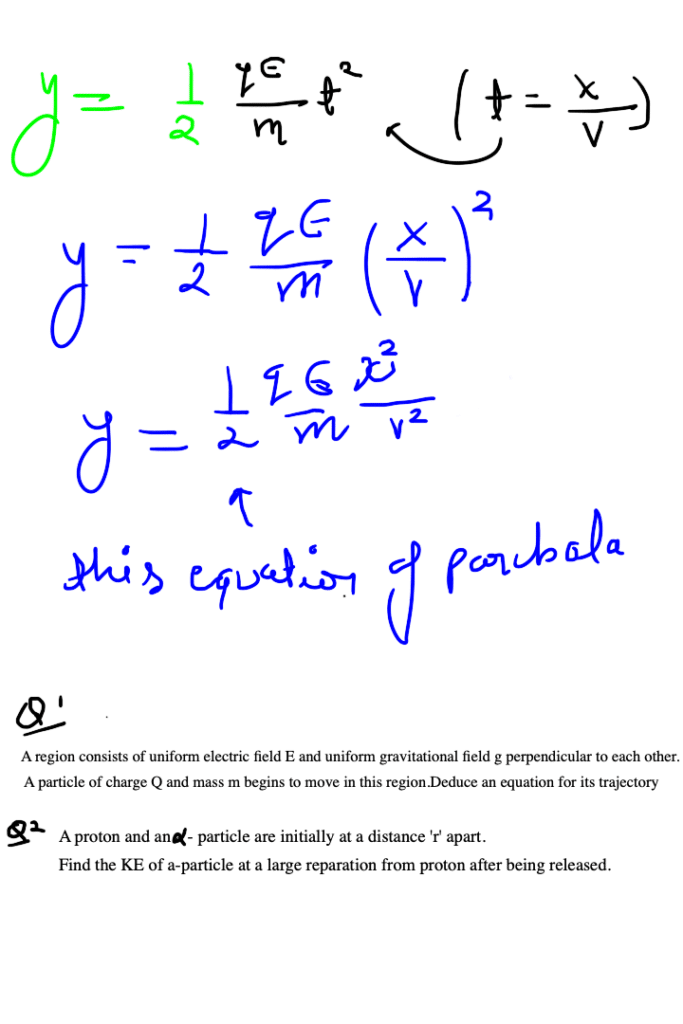ELECTRIC DIPOLE
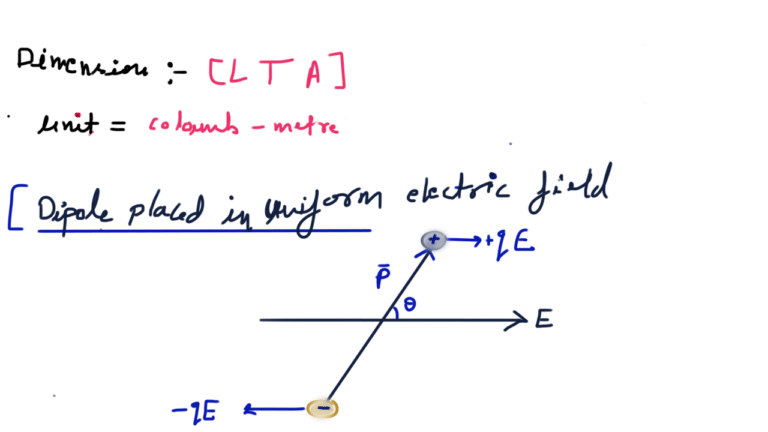
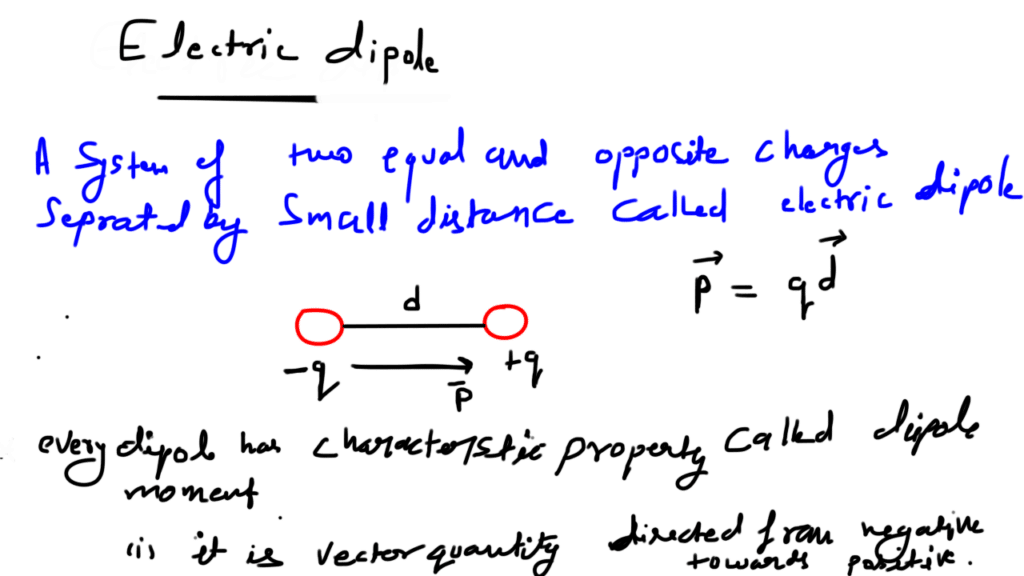
A system of two equal and opposite charges separated by a small distance is called electric dipole, shown in figure. Every dipole has a characteristic property called dipole moment. It is defined as the product of magnitude of either charge and the separation between them charges, given as
In certain molecules, the centres of positive and negative charges do not coincide. This results in the formation of electric dipoles. Atom is non – polar because the centres of positive and negative charges in it coincide. Polarity can be induced in an atom by the application of electric field. in that case it is called as induced dipole.
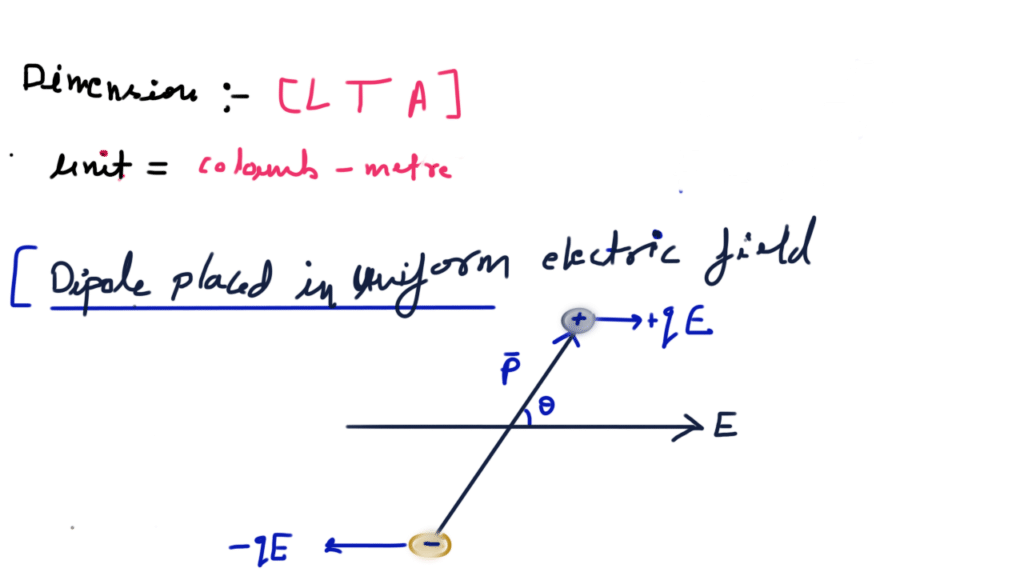
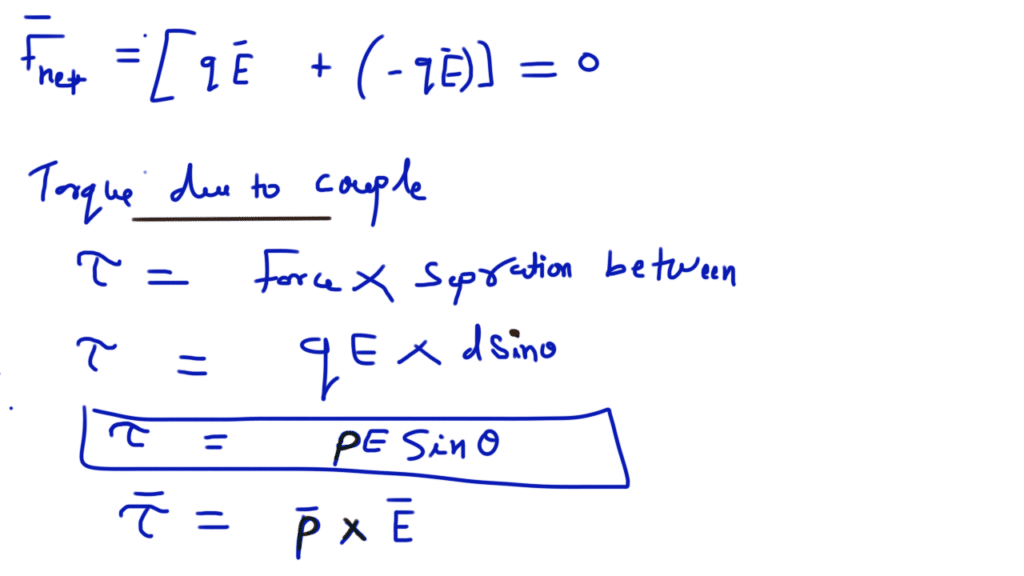
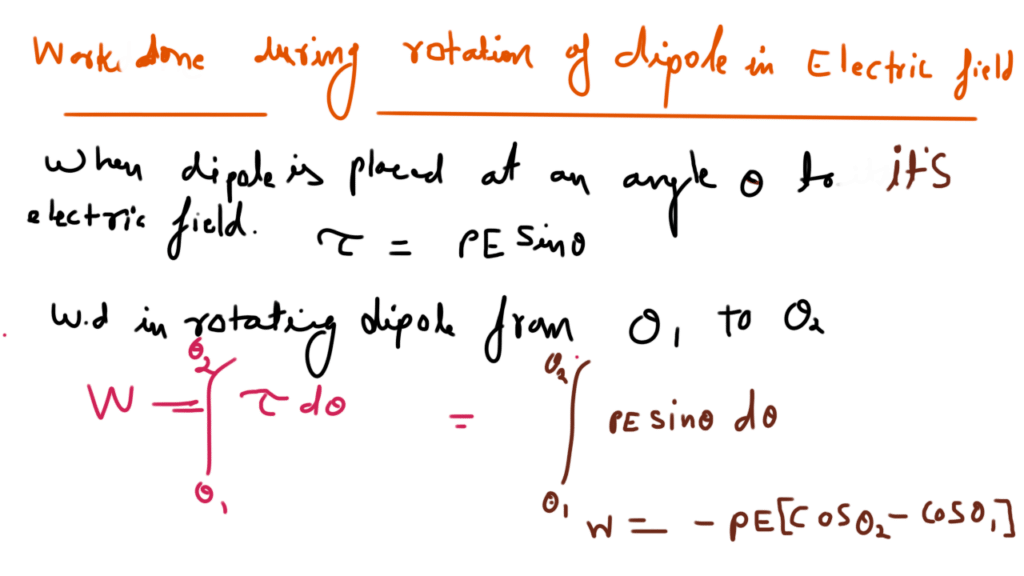
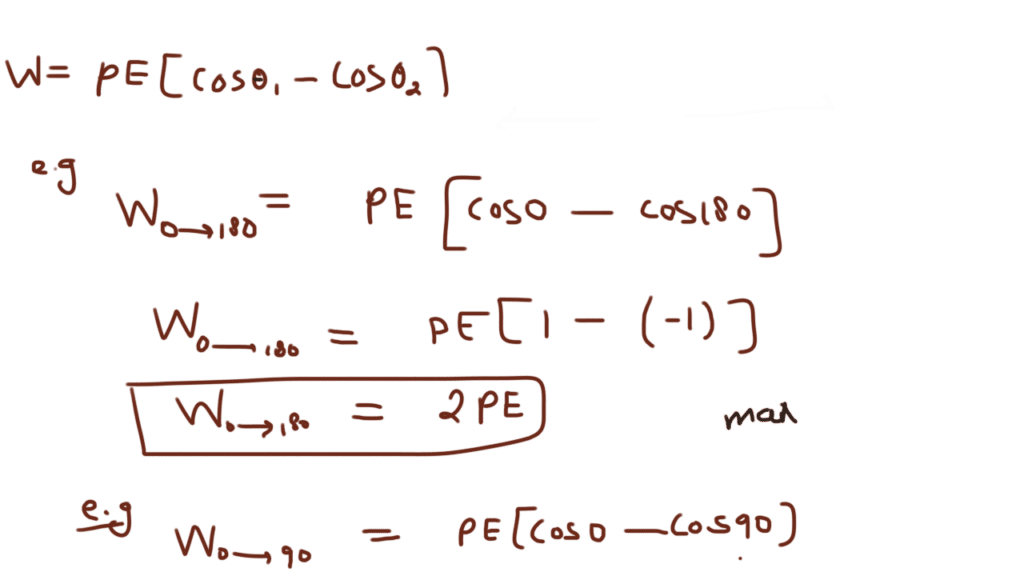

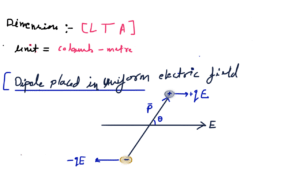
Electrostatic potential energy of a dipole placed in a uniform field is defined as the work done in rotating a dipole from a direction perpendicular to the field to the given direction i.e.
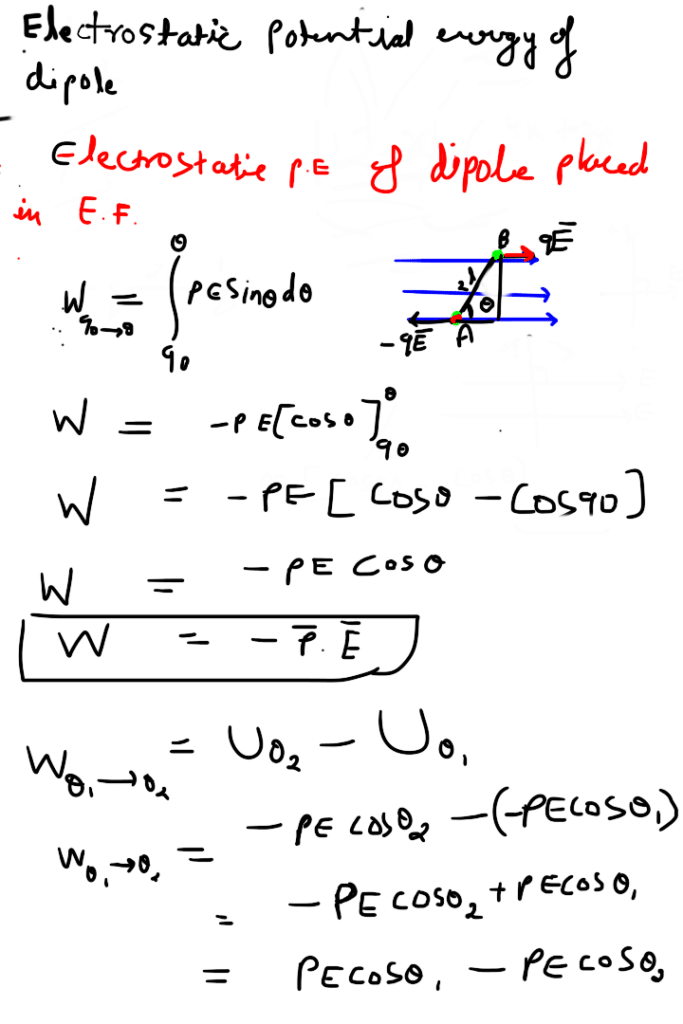
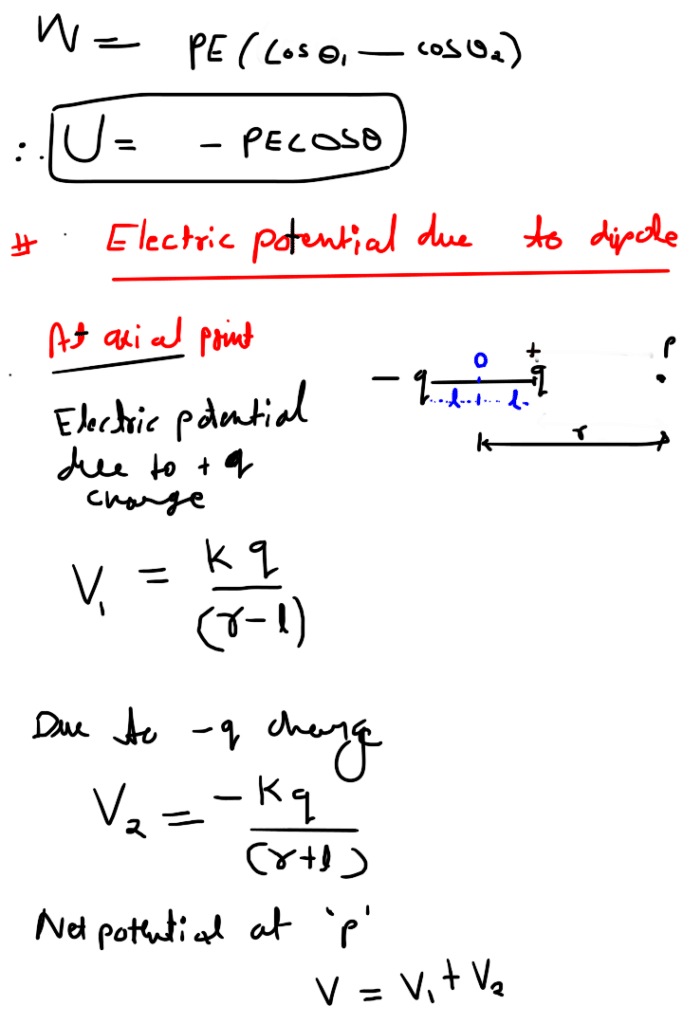
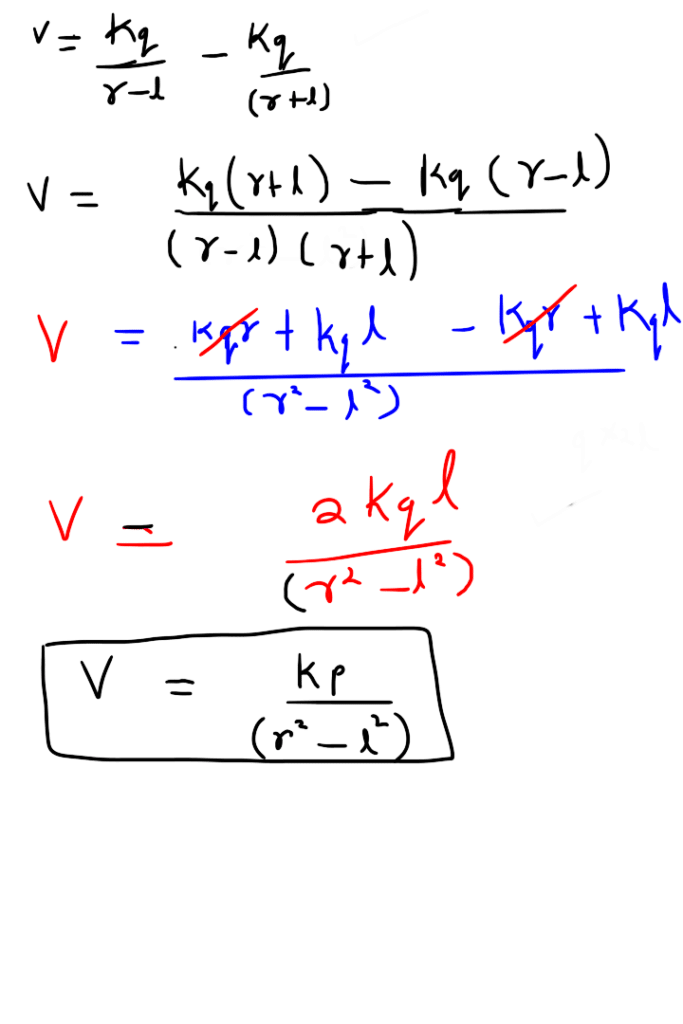
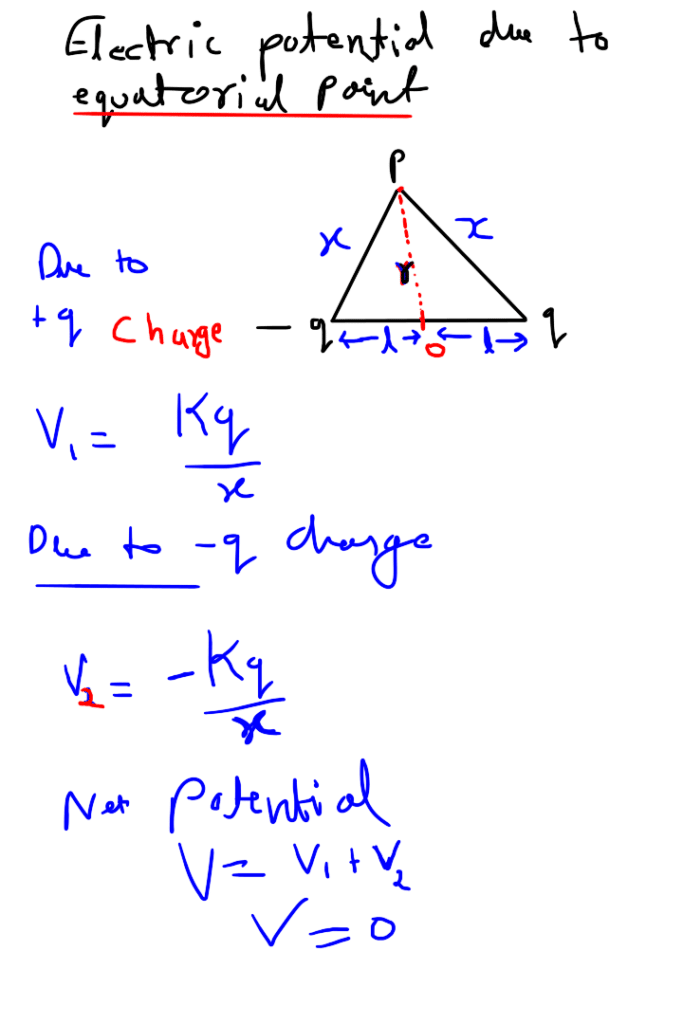
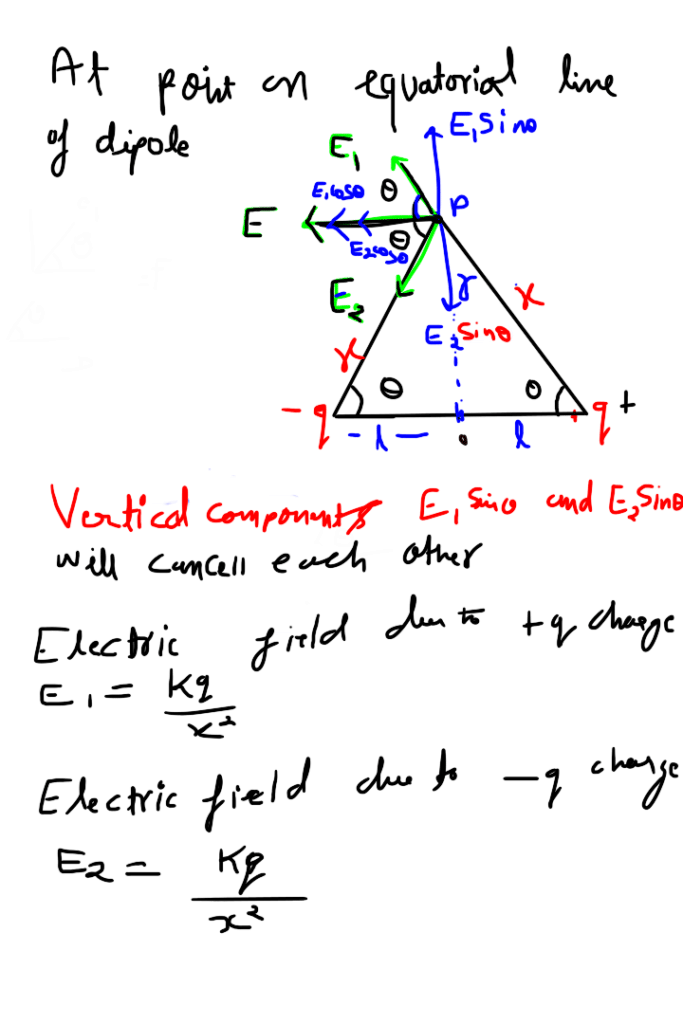
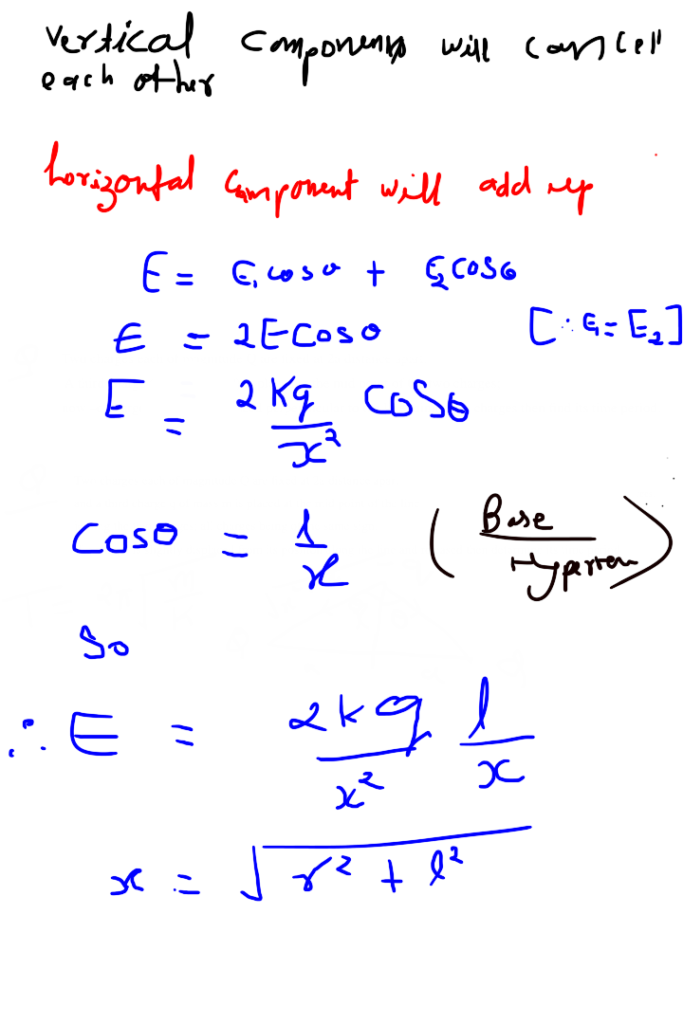
Electric potential due to equatorial point